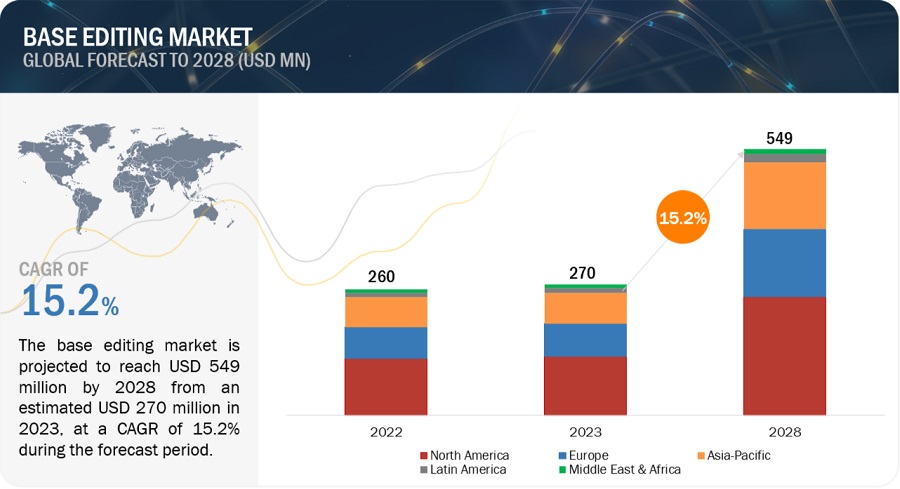
The base editing market, valued at $270 million in 2023, is projected to grow to $549 million by 2028, with a robust compound annual growth rate (CAGR) of 15.2%. This growth is driven by the increasing prevalence of genetic disorders and a growing recognition of the role of genetic mutations in various diseases, fueling the demand for precise gene editing technologies.
Market Dynamics
Driver: Rising Prevalence of Rare Diseases The surge in rare and orphan diseases has intensified the need for innovative therapies. Gene editing technologies, particularly base editing, are becoming crucial in addressing these conditions. Since many rare diseases are caused by genetic mutations, base editing is playing a key role in developing effective treatments. This trend is expected to significantly boost the base editing market as researchers and medical professionals seek novel solutions for these complex diseases.
Restraint: Ethical and Safety Concerns Despite its precision compared to traditional CRISPR-Cas9 methods, base editing technology raises ethical and safety concerns. Unintended genetic alterations can lead to unforeseen consequences, such as off-target effects. These risks pose challenges to the widespread adoption of base editing. Addressing these concerns through improved safety protocols and transparent dialogues is essential to advancing the technology while maintaining ethical standards.
Opportunity: Expanding Gene Therapy Pipeline and Regulatory Approvals The growing pipeline of gene therapy products presents significant opportunities for the base editing market. With over 1,000 gene therapy products in development and more than 200 in clinical trials, there is potential for transformative breakthroughs in treating genetic disorders. The base editing market stands to benefit from these advancements, particularly as gene therapy products continue to receive regulatory approvals.
Challenge: Off-Target Effects Off-target effects remain a significant challenge in base editing. Even minimal unintended modifications can lead to adverse outcomes, including new mutations or disruptions in gene function. Enhancing the specificity of base editors is an ongoing challenge, with researchers focusing on refining design and engineering to minimize these risks.
Market Ecosystem
The base editing ecosystem includes pharmaceutical and biotechnology companies, academic and research institutes, contract research organizations, and various service and product providers. These stakeholders collaborate to advance base editing technologies, enhance drug discovery, and develop new therapeutics.
Product and Service Segmentation
In 2022, the platform segment led the market, with base editing platforms being crucial for therapeutic genome editing. The Cytosine base editor segment is anticipated to grow rapidly due to its ability to model disease-causing mutations more effectively.
Regional Insights
North America dominated the base editing market in 2022, supported by a strong research infrastructure and favorable regulatory environment. Europe follows, with significant contributions to market growth.
Key Players
Notable players in the base editing market include Danaher Corporation, Merck KGaA, Revvity, Maravai LifeSciences, GenScript, Beam Therapeutics, Intellia Therapeutics, Cellectis, ElevateBio, Creative Biogene, and others.
Recent Developments
- May 2023: Revvity signed a licensing agreement with AstraZeneca for its Pin-point base editing system, enhancing cell therapies for cancer and immune-mediated diseases.
- July 2022: Beam Therapeutics collaborated with Verve Therapeutics to advance liver-mediated cardiovascular disease targets.
- February 2022: Intellia Therapeutics acquired Rewrite Therapeutics, expanding its genome editing toolbox with novel DNA writing technologies.
